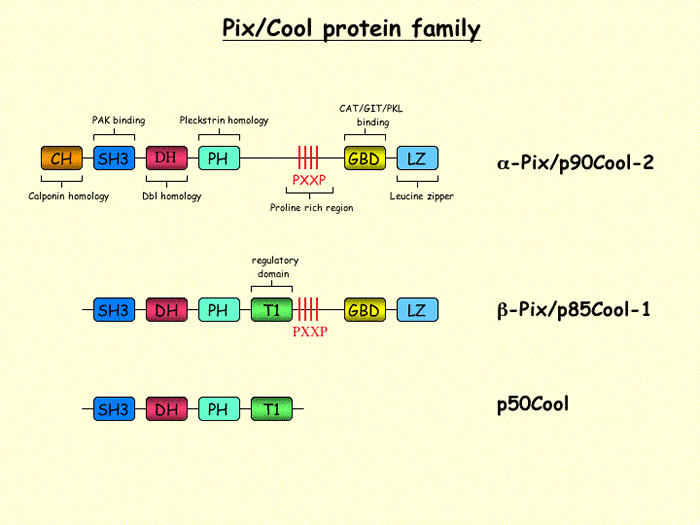| Ulrike Rennefahrt | ||||||
| home back | ||||||
The Rho family small GTPases have emerged as key regulators that mediate extracellular signaling pathways leading to the formation of actin-containing structures such as stress fibers, membrane ruffles, lamellipodia and filopodia. Besides changes in the cytoskeletal architecture of cells these GTPases also mediate biologic events like for instance the stimulation of DNA synthesis, cell cycle progression or cellular transformation. Rho GTPases cycle between inactive guanosine diphosphate (GDP)ñbound and active guanosine triphosphate (GTP)ñbound forms. The molecular transition between these two forms is regulated by guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs). The Pix (PAK-interacting exchange factor)/Cool (cloned out of library) proteins kinase represent one subgroup of the Dbl family of Rho-GEFs catalyzing the GDP-GTP exchange of Rac1 and Cdc42. So far, three members of this family have been described in more detail: p50Cool1, a longer splice variant from b-Pix/p85Cool1, and a distinct gene product called a-Pix/Cool-2. Pix proteins are ubiquitously expressed and contain the Dbl homology (DH) domain that is responsible for their catalytic activity. They can be activated in various ways, including phosphorylation and through the formation of homodimers mediated by a leucine zipper at the C-terminus of Pix. In addition, complex formation with a number of proteins (i.e. PAK, Rac/Cdc42, GIT, Shank, FAK, Cbl, Cat, etc.) could also regulate Pix activity and its biological function. Recently we identified a new serine phosphorylation site in Pix and with the help of various cell-based experiments we are exploring the physiological function of this phosphorylation. We are also investigating the putative cooperating role of Pix and PAK in cellular transformation.
|
||||||